Polymer Display and Energy Materials Research Group Publishes A Paper on PNAS, A Journal of National Academy of Sciences
In recent years, the polymer display and energy materials of the SMSE has worked with the Harvard University and Shanghai 6th People’s Hospital to achieve a series of research progress in applying new advanced functional materials in combination therapy of cancer. Lately, the group has made another new important progress in the design and preparation of a new advanced functional material and its application in the field of biomedicine. The relevant study has been published on PNAS (a journal of the National Academy of Sciences), a prestigious international academic periodical.
In the biomedical system, using biodegradable functional polymer capsule in the drug combination therapy system with high efficiency and low side effect is the key to the effective treatment of cancer and advanced biomedical applications. Drug combination therapy system has synergistic effect, which can effectively reduce drug resistance and drug dosage. Therefore, the preparation of novel biocompatible nano-functional materials and their applications in biomedicine and cancer treatment have always been a research hotspot in the fields of materials science and life science. At the same time, the research and development of photothermal multifunctional nano polymer capsule materials loaded with anticancer drugs or antibodies is of important theoretical and practical significance.
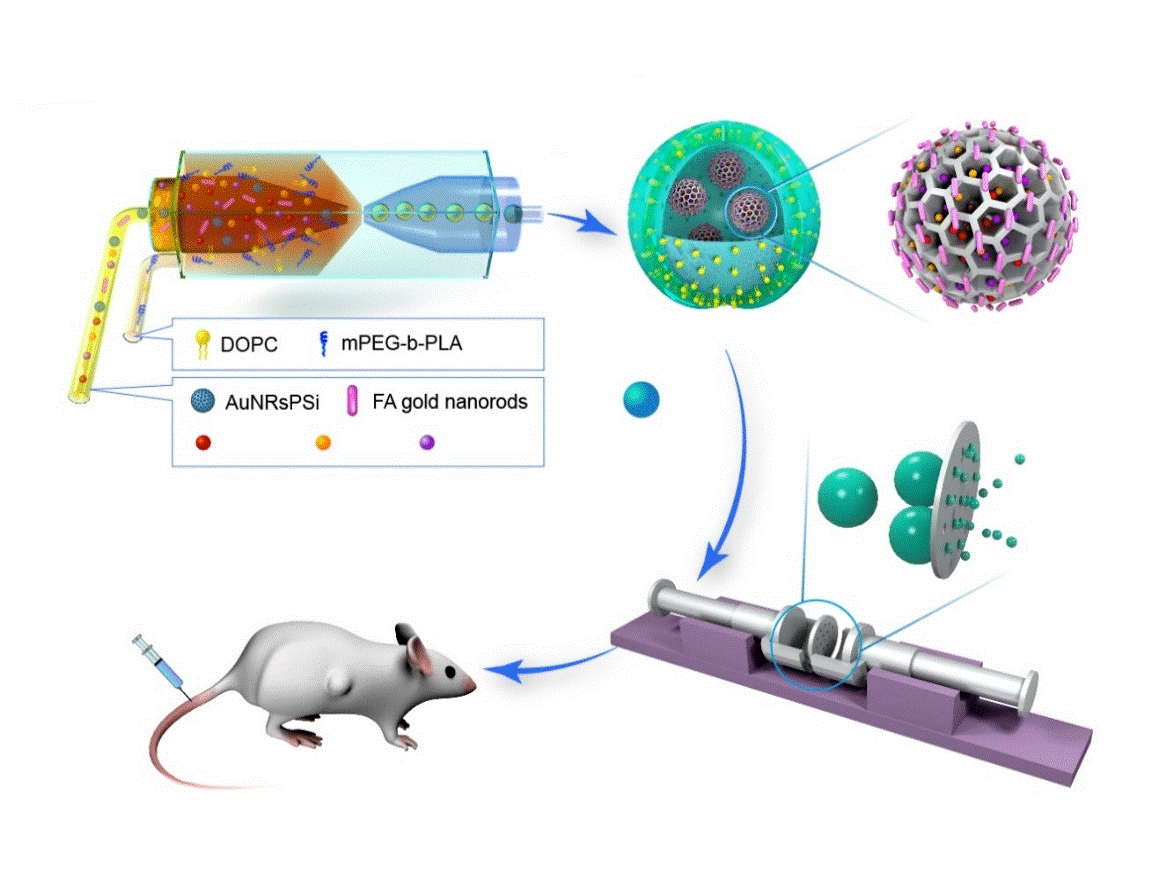
Scheme 1. The fabrication of photothermal responsive nanosized hybrid polymersome as versatile therapeutics co-delivery nanovehicle for intravenous injection cancer treatment.
To solve these key problems, the group designed and prepared gold nanorods conjugated porous silicon nanoparticles with photothermal property and loaded with hydrophobic anticancer drugs, and encapsulated it in polymer capsule nanovehicles through microfluidic technology-membrane extrusion technology for the loading of molecular target drugs, chemical drugs or immunosuppressive agents. At the same time, it was successfully used in the biomedical and chemical application of intravenous injection therapy of HER2-positive breast cancer. Gold nanorods conjugated porous silicon nanoparticles have excellent photothermal property, and can increase the loading efficiency of hydrophobic anticancer drugs. The gold nanorods conjugated porous silicon nanoparticles encapsulated in polymer capsule core-shell structure nanovehicles can effectively control the release of drugs. The combination of different anticancer drug systems coloaded in the new functional material can effectively inhibit drug resistance. With excellent biocompatibility and photothermal effect, the functional material represents an ideal system for drug loading and material delivery, as it can promote more effective tumor suppression and cancer cell death and reduce drug resistance and side effects through synergism of drugs and targeting effect of drugs and synergism between drugs and antibodies. This study has drawn wide attention and recognition among peers at home and abroad. This work has been published on PNAS (2019, 116, 7744-7749), a journal of the National Academy of Sciences.
Photothermal-responsive nanosized hybrid polymersome as versatile therapeutics codelivery nanovehicle for effective tumor suppression
Hongbo Zhang, Wenguo Cui, Xiangmeng Qu, Huayin Wu, Liangliang Qu, Xu Zhang, Ermei Mäkilä, Jarno Salonen, Yueqi Zhu, Zhou Yang, Dong Chen, Hélder A. Santos, Mingtan Hai, and David A. Weitz
PNAS April 16, 2019 116 (16) 7744-7749; first published March 29, 2019 https://doi.org/10.1073/pnas.1817251116
Edited by Tobin J. Marks, Northwestern University, Evanston, IL, and approved March 6, 2019 (received for review October 10, 2018)
· Author contributions: M.H. and D.A.W. designed research; H.Z., W.C., X.Q., L.Q., X.Z., E.M., J.S., Y.Z., and D.C. performed research; H.Z., W.C., X.Q., L.Q., X.Z., E.M., J.S., Y.Z., Z.Y., D.C., H.A.S., and M.H. analyzed data; and H.Z., H.W., H.A.S., M.H., and D.A.W. wrote the paper.
· The authors declare no conflict of interest.
· This article is a PNAS Direct Submission.
· To whom correspondence may be addressed. Email: zhuyueqi@hotmail.com, mingtanhai@mater.ustb.edu.cn, or weitz@seas.harvard.edu


